The Delicate Dance of Woad's Chemistry
All Indigos including Woad, Indian Indigo, Tyrian Purple of the Murex snail and numerous other lesser quality plant sourced indigo, require the same complex balance of chemistry to occur in order to dye. The blue pigment indigotin, which is extracted from these different sources, is an insoluble substance that is not able to be dissolved in water. Therefore this indigotin needs to be converted into a form which can be dissolved to enable it to work as a dye.
The indigotin must be changed from either blue, or Tyrian purple, into the water soluble white indigotin. This involves removing all of the oxygen from the solution in the dye vat by a process of reduction. What you see is the blue or purple changing to a yellow liquid. In ancient times this was done by fermentation, while now we often use chemicals. Both methods require that the vat be maintained in an alkaline state in order for the removal of the oxygen to be successful. There are substances used such as lye and soda ash to maintain that alkalinity. The vat must be kept warm at all times, between 45-60C or 110-140F. The surface area of the liquid requires protection from having oxygen introduced back in to the solution. Stirring is very gentle and only if really needed.
Materials for dyeing are added, soaked and removed from the vat very gently, again to lessen the amount of oxygen being reintroduced. When they emerge from the vat they are a watery yellow, but begin to turn blue, or purple, if Tyrian Purple, upon coming in contact with the air! This re oxidation process causes the dye to be bonded to the fabric. In the case of Woad, this is a permanent bonding, making it completely colour fast.
The Tyrian Purple dye from the Murex snail needs an extra step in order to give a blue colour, rather than it's original purple. It's indigo molecule has 2 bromine atoms attached, red pigment causing it to be purple, that the plant based indigo molecules don't have.
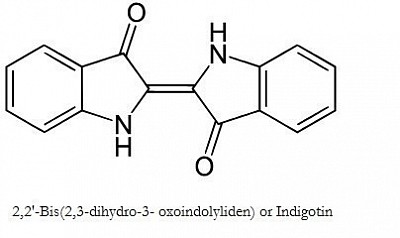
Chemical formula of Woad, Indigo and other plant indigos. The Murex snail dye becomes this once the 2 Bromine atoms have been removed using ultraviolet light
This is accomplished by exposing the solution in a clear glass vessel to ultraviolet light, while it is being reduced. This exposure knocks the 2 bromine atoms off the Indigo molecule. This changes the molecule to now be chemically identical to the plant indigos' and giving a blue dye.
The chemistry pictures of the Woad and Murex molecules, are from here.




